Ethylene oxide as a vapor and water as liquid
Ethylene oxide as a vapor and water as liquid, both at 25°C and 101.33 kPa, react to form a liquid solution containing ethylene glycol (1,2-ethanediol) at the same conditions: Answer:13.39Phase-equilibrium equations: Ethylene oxide(1): p1 = y1∙P = 415∙x1 P := 101.33∙kPa
Water (2): x2∙Psat2 = y2 ∙ P Psat2 : = 3.166∙kPa
Ethylene glycol(3): Psat3 = 0.0 y3 = 0.0 Therefore, y2 = 1 - y1 and x3 = 1 - x2 - x3 For the specified standard states: (CH2)2O (g) + H2O(l) = CH2OH.CH2OH(l) By Eq. (13.40) and the stated assumptions,
T:= 298.15∙kelvin Data from Table C.4:
k = 6.018x1012 So large a value of k requires either y1 or x2 to approach zero if y1 approaches zero, y2 approaches unity, and the phase-equilibrium expression for water (2) makes x2 = 32, which is impossible. Thus x2 must approach zero, and the phase-equilibrium equation requires y2 also to approach zero. This means that for all practical purposes the reaction goes to completion. For initial amounts of 3 moles of ethylene oxide and 1 mole of water, the water present is entirely reacted along with 1 mole of the ethylene oxide. Conversion of the oxide is therefore 33.3 %. 谢谢亲的回惠顾,期待您的下次光临! 添加客服微信:bbwxnly,购买沟通交流so easy 欢迎咨询以下我们的一站式服务内容:课后习题解答查题|文件文档解锁下载|会员帐号包月月卡 Bartleby|Bookrags|brainly.com|Coursehero|Chegg|eNotes|Ebook|gradebuddy|Grammerlly|Numerade |QuillBot|Oneclass|Studypool|SaveMyEaxms|Studymode|ScholarOne|SlideShare|SkillShare|Scribd|SolutionInn|Study.com|Studyblue|Termpaperwarehouse|and more… 谢谢亲的支持,祝您学习愉快:) |


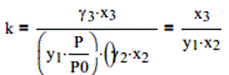


评论
发表评论